Deep-tech Bengaluru-based healthcare company: Niramai Health Analytix receives US FDA clearance for medical device SMILE-100 System
- ByStartupStory | March 14, 2022

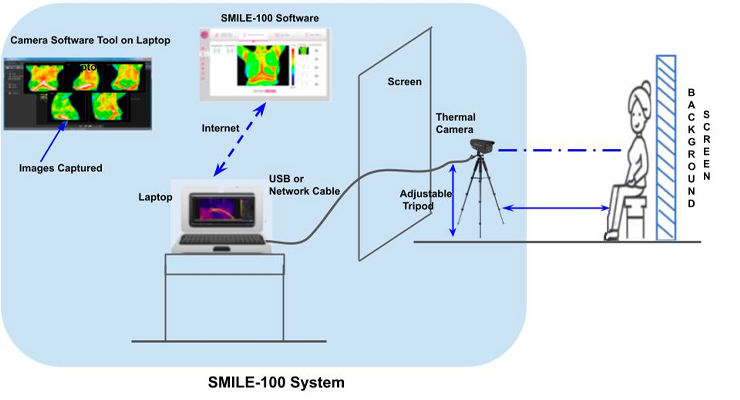
The deep-tech Bengaluru-based healthcare company, Niramai Health Analytix, has received US FDA clearance for their first device, SMILE-100 System which offers a novel radiation-free, non-touch, accurate, breast cancer screening solution in India.
In order to review, measure and analyse thermally significant indications in the breast region, the healthcare personnels have been assisted by the breast thermography device. The device can be used in the hospital, outpatient surgery, acute care settings, healthcare practitioner facilities or an environment where patient care is provided with qualified healthcare personnel.
According to a statement, the SMILE-100 System, helps experts to visualise high thermal activity patterns clearly demarcated on thermal images as hotspots. It can also enable healthcare professionals in order to make better decisions in breast cancer screening and diagnosis. Patented AI-based algorithms are also used in SMILE-100 in order to check the quality of input thermal images, which can reduce the errors in thermal image capture and also help to confidently perform the imaging to low skilled health workers.
Dr. Geetha Manjunath, CEO and Founder of Niramai, said that they are super excited to announce the FDA 510(k) clearance of SMILE-100 System. It is a proud moment for the team. To go through the very rigorous US regulatory process was a great learning experience.
NIRAMAI was co-founded by Dr. Geetha Manjunath and Ms Nidhi Mathur in July 2016. A total of $7 million has been raised so far from institutional investors from India, Japan and Singapore. Dream Incubator, Ankur Capital, Axilor Ventures, BeeNext, pi Ventures, 500 Startups, and others are also included as the investors. European CE approval for their Thermalytix™ Solution was announced by the company last year and pilots have already been started in multiple European countries. Niramai, with this FDA approval will get a tool to foray into the US market and beyond.
Mr. Manish Singhal, Founding Partner of pi Ventures and the lead investor of Niramai who has been part of their journey since 2017 also commented that in the last five years, they have seen Niramai growing in every aspect such as product innovation, team strength, clinical provenance, product adoption and international recognition. They congratulated the team for the exciting win of the FDA clearance for their SMILE-100 System. They said that it is a great feat for any Indian Startup to get the US approval for their medical device. They further added that this can be the key inflection point for the company in order to make their product truly global and saving lives all over the world.









